Products
E&S Healthcare promises a healthier life for mankind.
DxMe® BC
DxMe® BC
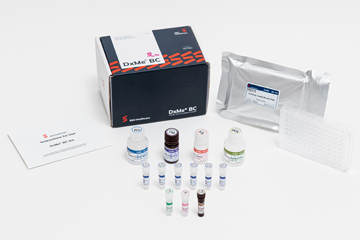
Blood-based breast cancer in vitro diagnostic kit using ELISA
The current gold standard of early detection of breast cancer
is mammography that has inevitable weak points such as cost, discomfort, and
radiation exposure. Besides, an additional examination might be necessary for
better diagnosis of dense breasts.
Our breast cancer diagnostic kit, DxMe® BC, measures a specific
protein biomarker from the blood by ELISA regardless of breast conditions. It
can help detect breast cancer exactly. The accuracy can be maximally enhanced
when it is accompanied with mammography.
Specifications
| Item | Particular |
|---|---|
| Test Format | Sandwich ELISA |
| Brand Name | DxMe® BC Kit |
| Specificity | 96.43%1 |
| Sensitivity | 97.32%1 |
| Cut-off | 11.4 ng/ml1 |
| 99% Reference range | 3.4 ~ 18.8ng/ml |
| Detection Range | 3.4 ~ 44.4ng/ml |
| Tests per kit | 88 Tests |
| QMS Certificates | ISO 13485 KGMP BGMP |
| Product registration | European union Malaysia Serbia New Zealand |
| Stability | ≤ 12 months (at 4℃) |
- Specificity : Ability to distinguish normal group from the tester group
- Sensitivity : Ability to distinguish abnormal group (patient group) from tester group
- Cut-off-Value : Reference value for distinguishing between normal and abnormal groups
Brochure
Features
.jpg)
- 1 The blood Trx1 level could correct mis- or incompletely judged mammography with 93% efficency1.
- 2 When mammography and Trx1 level were analyzed together, the accuracy was near 100% 1.
.jpg)
- 1 The levels of blood Trx` were measured from 308 biopsy-confirmed BC patients, 82 breast benign tumor bearers, and 254 woman without any disease (total of 336 women as a control group)1.
- 2 The clinical sensitivity and specificity were 96.43% and 97.32%, respectively.
- 3 The AUC was 0.985 (95% CI: 0.972 to 0.993)1.
- 4 The level of blood Trx1 could differentiate BC regardless of characteristics of BC including stage 1, 2.
.jpg)
- 1 The blood Trx1 level was decreased dramatically after surgery, and it was likely to depend on the presence or absence of a tumor mass3.
- 2 The blood Trx1 level has potential as a monitoring biomaker to assist the management of BC patients after treatment 3.
Clinical evidence
Presentation
Scientific Articles
Reference
- 1 Kim, Y. (2022, July). Clinical Evaluation of Thioredoxin 1 in the Blood as a Novel Biomarker to Detect Breast Cancer. Poster presented at American Association for Clinical Chemistry annual scientific meeting & Clinical Lab Expo, Chicago, IL, U.S., Abstract retrieved from https://meeting.aacc.org/-/media/Files/Meetings-and-Events/Annual-Meeting/2022/AACC22_AbstractBook_Final.pdf?la=en&hash=B978AA002D9D9DE1E12185BCB2CCB3861302F210
- 2 Lee, Y.J., Kim, Y., Choi, B.B., Kim, J.R., Ko, H.M. Suh, K.H. & Lee, J.S. (2022). The blood level of thioredoxin 1 as a supporting biomarker in the detection of breast cancer. BMC Cancer, 22:12
- 3 Ko. H.M. (2022, April). The Blood Level of Thioredoxin 1 as a Biomarker for the Monitoring of Breast Cancer Patients Underwent Surgery. Poster presented at Global Breast Cancer Conference, Seoul, KR, Abstract retrieved from http://gbcc.kr/upload/GBCC%202022_Announcement.pdf
Breast Cancer
-
No. 1
Gynecologic cancer in 1 out of 5 women
-
70%
Incidence of women
in their 40s/50s -
X4
Sharp increase over 15 years
(As of 2017)
-
Over
90%Early detection survival rate
-
51.9
median age of patient
(KBCS 2020)
The Statistics

Incidence & Mortality
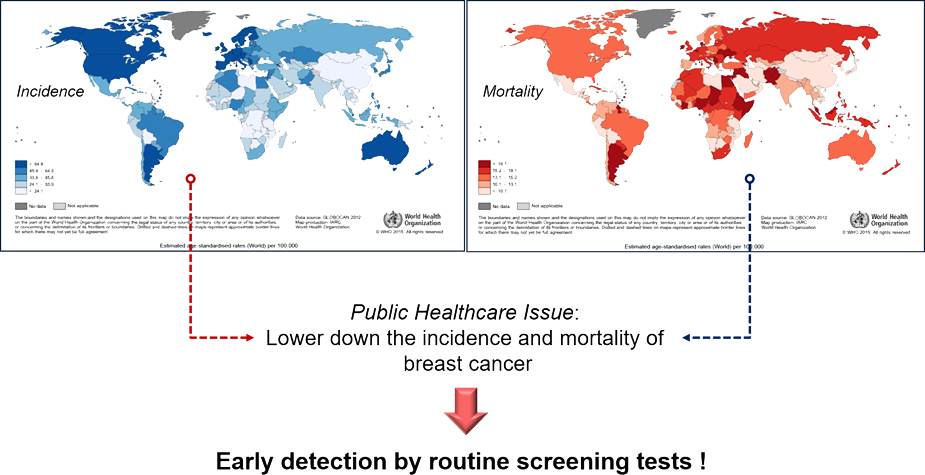
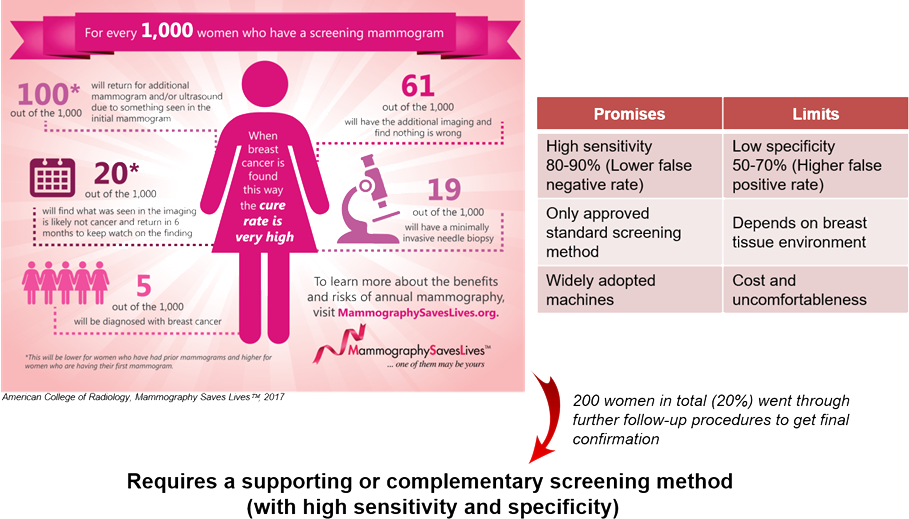
Golden Standard(Mammography)
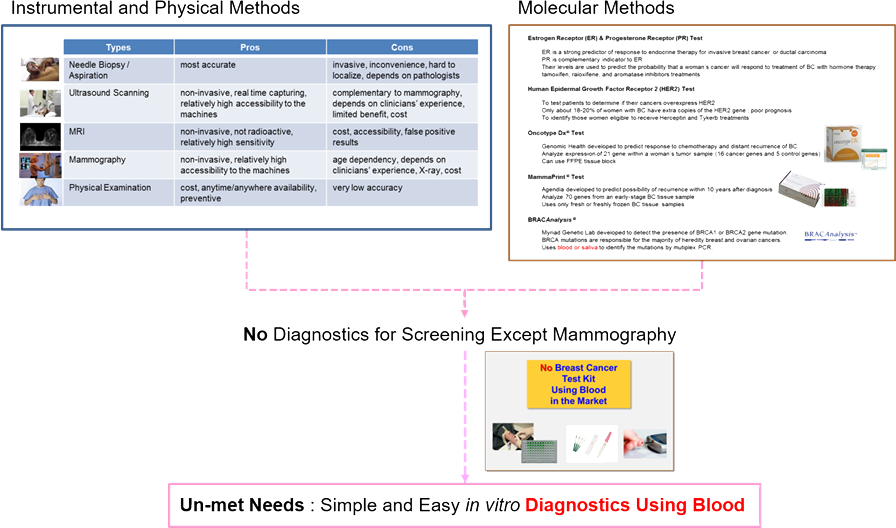
Current Diagnostics
.png)